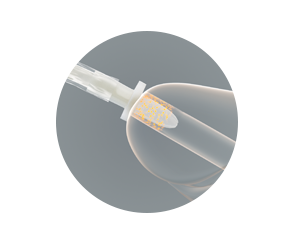
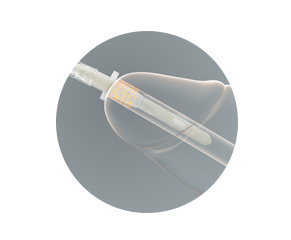
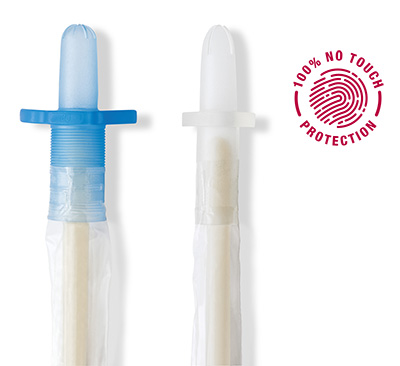
The protective tip and sleeve are why all VaPro™ catheters provide 100% No Touch Protection.
The protective tip
- Helps shield the sterile catheter during insertion from bacteria located within the first 15mm of the distal urethra
- Helps reduce the risk of carrying bacteria into the urinary tract
The protective sleeve
- Allows for catheter to be gripped anywhere
- Provides a barrier that helps keep germs away from the catheter
VaPro™ catheters are easy to use
Ready to use right out of the package with no extra steps required

Designed with smooth rounded eyelets to enhance user comfort during insertions and withdrawal

Packaging designed with finger holes to be easy to open

Pocket-sized packaging for easy transportation and discreet out of home usage
Our Products
Learn more about the wide variety of VaPro™ 100% No Touch Protection products.